By Jim English
 It is estimated that 240 million people worldwide suffer 1.4 billion migraine attacks each year. Current therapeutic options are often not effective or are accompanied by troublesome side effects. New research reveals that an extract of the ancient medicinal plant, Petasites hybridus (butterbur), can reduce the frequency, duration and pain of migraine attacks by up to 60% with a degree of safety and tolerance superior to most pharmaceutical drugs.
It is estimated that 240 million people worldwide suffer 1.4 billion migraine attacks each year. Current therapeutic options are often not effective or are accompanied by troublesome side effects. New research reveals that an extract of the ancient medicinal plant, Petasites hybridus (butterbur), can reduce the frequency, duration and pain of migraine attacks by up to 60% with a degree of safety and tolerance superior to most pharmaceutical drugs.
Migraine
Migraines strike women three times as frequently as men, affecting 5% of men and 17% of women in the United States. (1,2) In direct medical costs and lost productivity, the annual cost in the United States is estimated to exceed $17 billion. (3) In spite of these numbers, according to the International Headache Society many migraines go unrecognized and undertreated due largely to the fact that there are no recognized biological markers to confirm the diagnosis. (4,5,6)
While the exact cause of migraine is not fully understood, migraines can be triggered by internal and external factors, such as stress, anxiety, food allergies, hormones and environmental changes, such as light, heat and altitude. When triggered, the nervous system responds with increased serotonergic activity in the brain, vasodilation of the extra-cranial blood vessels, and concomitant vasoconstriction of the intra-cranial blood vessels. (7)
Migraine Symptoms
Unlike tension headaches, migraines are a full-blown neurologic disorder characterized by recurrent attacks of headache that, if not successfully treated, can last anywhere from 2 to 72 hours. Migraine pain is usually unilateral, appearing as a throbbing, pounding pain on only one side of the head. During different phases of a migraine attack, pain can move from one part of the head to another, or radiate down into the neck and the shoulders. Skin hypersensitivity, including scalp irritation and tenderness, also occur frequently during a migraine attack.
Migraine sufferers often experience nausea and vomiting accompanied by a pronounced and extreme sensitivity to light (photophobia), sounds (phonophobia) and odors (osmophobia). In severe attacks, even normal activities such as standing or walking can intensify pain to the point where normal function is completely disrupted.
Migraine Aura
Approximately 20% of all migraine sufferers experience visual disturbances that are referred to as the migraine aura. In addition to alterations in perception of light, sound and smell, aura can include bizarre visual distortions such as blurry vision, spots, flashing lights, wavy lines, flashing lights, and/or partial loss of sight. Other symptoms may include vertigo, tingling or numbness of the face or extremities, auditory hallucinations, difficulty or confusion when forming words, and impaired hearing. Some patients even have the aura without the headaches.
Standard Migraine Treatment
Standard treatments aim to control symptoms by calming sensitive nerve pathways and reducing the inflammatory response while preventing future attacks. As with tension headaches, some individuals find that very mild migraine attacks can be treated with standard analgesics such as aspirin, acetaminophen, ibuprofen and naproxen. These drugs, in addition to their potential gastrointestinal side effects, have been shown to increase risk of rebound (medication-induced) headaches. (8) Combination medications such as acetaminophen with codeine, aspirin with codeine and caffeine, and aspirin with butalbital and caffeine (with or without codeine) are sometimes used. However, overuse of combination medications is one of the most prominent causes of rebound headache, which is the leading form of chronic daily headache. (7)
5-HT1 Receptor Agonists
Sumatriptan, a selective 5-hydroxy-triptamine 1 (5-HT1) receptor agonist, has been shown more effective than placebo in treating moderate to severe migraines (50-80% versus 20-40%) when administered by subcutaneous injection. Reported side effects include tightness in the chest, chest pain, pain in the throat, tingling in the head or limbs, nausea, and a high rate (44%) of migraine recurrence within 24 hours. (9,10)
Dihydroergotamine (DHE), a nonselective 5-HT1 receptor agonist, has also been shown effective in relieving headache when used subcutaneously, intramuscularly or intravenously, but side effects are similar to those of sumatriptan. (11,12) Ergotamine has also been used for many years to treat migraine, but meta-analysis has demonstrated little benefit from oral dosing. (13) Side effects of ergotamine resemble those of DHE, but nausea is usually more severe. (14,15,16)
Other migraine medications include:
- Beta-blockers (Propranolol)
- Tricyclic anti-depressants (Amitriptyline)
- MAO inhibitors (Phenelzine)
- Adrenergic blockers (Clonidine)
- Serotonin antagonists (Cyproheptadine)
- Calcium channel antagonists (Verapamil)
- Anticonvulsants (Phenytoin)
- Butorphanol nasal-spray (Stadol)
As with the previous medications, side effects of these medications are substantial. In the case of beta-blockers, they are contraindicated for patients with asthma, chronic obstructive pulmonary disease, insulin-dependent diabetes mellitus, heart failure, or peripheral vascular disease. Calcium-channel blockers, which can take several months to become effective, are contraindicated in pregnancy and in patients with hypotension, congestive heart failure, arrhythmias or depressive illness. (17,18,19,20)
Petasites hybridus
Petasites hybridus (butterbur) is an herbal plant found throughout Europe, Asia and North America. For centuries the leaves and rhizomes of this perennial shrub have been used as an important medicinal herb. (21,22) Modern researchers have discovered that extracts of petasites contain active ingredients that prevent migraines and act as an antispasmodic to support chronic cough or asthma. (23)
Active Constituents
The main active ingredients of petasites hybridus are two sesquiterpenes, petasin and isopetasin. Research has shown that petasin possesses anti-spasmodic properties that help to reduce spasms in smooth muscle and vascular walls. Petasin has also been found to be a powerful anti-inflammatory agent that inhibits synthesis of leukotrienes that act as potent pro-inflammatory agents in blood vessel walls, causing bronchoconstriction in asthma. (24)
The second active ingredient, isopetasin, has also been found to reduce inflammation by modulating prostaglandin metabolism. Together these ingredients have an antispasmodic effect on vascular walls, with a marked affinity for cerebral blood vessels. (25)
Petasites Extract Reduces Incidence of Migraine
Researchers in Germany conducted a randomized, placebo-controlled, double-blind clinical study using a standardized extract of petasites (Petadolex®). 60 patients suffering from headaches with and without aura randomly received either 50 mg of a standardized petasites extract or placebo, twice daily for 12 weeks. At the conclusion of the test the researchers found that, compared to placebo, petasites hybridus significantly reduced the frequency of migraine attacks and days with migraine per month, as well as the frequency of accompanying symptoms. Compared to baseline, petasites hybridus reduced the frequency of attacks by 46% after 4 weeks, 60% after 8 weeks and 50% after 12 weeks of treatment (placebo group: 24%, 17% and 10%, respectively). (26)
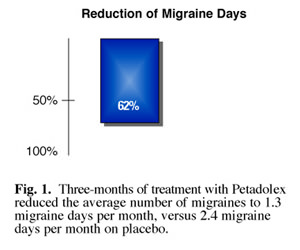
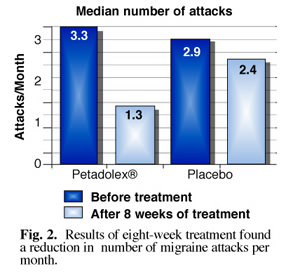
The researchers also found that the extract reduced the total number of migraine days (Fig. 1). Of particular interest was the finding that patients who entered the study reporting at least three migraine attacks per month showed the most pronounced reduction in events. (Fig. 2) Patients also reported significant alleviation of intensity of migraine pain, leading researchers to conclude that petasites extract is most effective in reducing migraine in patients who suffer the most from frequent severe attacks.
Conclusion
Petadolex® is a special standardized extract of Petasites hybridus that offers a new option for migraine sufferers. Petadolex is effective in reducing the number of days with migraines per month, in decreasing migraine-associated symptoms, and in reducing both the duration and intensity of migraine pain. Petasites extract has a history of excellent tolerance, and no adverse side effects have been reported. It should be noted that it is important to take Petadolex on a daily basis for maximum effectiveness.
References
1. Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence: a review of population-based studies. Neurology 1994;44(6 suppl 4):S17-S23.
2. Celentano DD, Stewart WF, Lipton RB, Reed ML. Medication use and disability among migraineurs: a national probability sample. Headache 1992;32:237-8.
3. De Lissvoy G, Lazarus SS. The economic cost of migraine: present state of knowledge. Neurology 1994;44(6 suppl 4):S56-S62.
4. Lipton RB, Stewart WF, Celentano DD, Reed ML. Undiagnosed migraine: a comparison of symptom-based and physician diagnosis. Arch Intern Med 1992;152:1273-8.
5. Headache Classification Committee, International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8(suppl 7):1-96.
6. Ad Hoc Committee on Classification of Headache. Classification of headache. JAMA 1962;179:717-8.
7. Mathew NT. Chronic daily headache: clinical features and natural history. In: Nappi G, et al, editors. Headache and depression: serotonin pathways as a common clue. New York: Raven Press; 1991:49-58.
8. Saper JR, Silberstein S, Gordon CD, et al. Handbook of headache management. Baltimore: Williams and Wilkins; 1993.
9. Subcutaneous Sumatriptan International Study Group. Treatment of migraine attacks with sumatriptan. N Engl J Med 1991;325:316-21.
10. Winner P, Ricalde O, Le Fortce B, Saper J, Margul B. A double blind study of subcutaneous dihydroergotamine vs subcutaneous sumatriptan in the treatment of acute migraine. Arch Neurol 1996;53:180-4.
11. Winner P, Ricalde O, Le Fortce B, Saper J, Margul B. A double blind study of subcutaneous dihydroergotamine vs subcutaneous sumatriptan in the treatment of acute migraine. Arch Neurol 1996;53:180-4.
12. Scherl ER, Wilson JF. Comparison of dihydroergotamine with metoclopramide versus meperidine with promethazine in the treatment of acute migraine. Headache 1995;35:256-9.
13. (Dahlof C. Placebo-controlled clinical trials with ergotamine in the acute treatment of migraine. Cephalalgia 1993;13(3):166-71.
14. Hakkarainen H, Allonen H. Ergotamine vs metoclopramide vs their combination in acute migraine attacks. Headache 1982;22:10-2.
15. Kangasniemi P, Kaaja R. Ketoprofen and ergotamine in acute migraine. J Intern Med 1992;231:551-4.
16. Kinnunen E, Erkinjuntti T, Farkkila M, et al. Placebo-controlled double-blind trial of pirprofen and an ergotamine tartrate compound in migraine attacks. Cephalalgia 1988;8:175-9.
17. Al-Qassab HK, Findley LJ. Comparison of propranolol LA 80 mg and propranolol LA 160 mg in migraine prophylaxis: a placebo controlled study. Cephalalgia 1993;13:128-31.
18. Ryan Sr RE, Ryan Jr RE, Sudilovsky A. Nadolol: its use in the prophylactic treatment of migraine. Headache 1983;23:26-31.
19. Gerber WD, Diener HC, Scholz E, Niederberger U. Responders and non-responders to metoprolol, propranolol and nifedipine treatment in migraine prophylaxis: a dose-range study based on time-series analysis. Cephalalgia 1991;11:37-45.
20. Markley HG. Verapamil and migraine prophylaxis: mechanisms and efficacy. Am J Med 1991;90(suppl 5A):48S-53S.
21. Eaton J. Butterbur, herbal help for migraine. Nat Pharm 1998;2:1,23-24.
22. Mauskop, A. Petasites hybridus: ancient medicinal plant is effective prophylactic treatment for migraine. Townsend Lett 2000;202:104-106.)
23. Grieve M. Butterbur. In: Leyel CF, ed. A Modern Herbal, electronic version. New York, NY: Dover Publications, Inc. 1971.
24. Thomet OA, Wiesmann UN, Schapowal A, Bizer C, Simon H. Role of petasin in the potential anti-inflammatory activity of a plant extract of petasites hybridus. Biochem Pharmacol 2001 Apr 15;61(8):1041-1047.
25. Altern Med Rev 2001 Jun;6(3):303-310. 26. Grossmann M, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Int J Clin Pharmacol Ther 2000 Sep;38(9):430-435.