By Jim English
According to the Centers for Disease Control (CDC), 61 million Americans – 25 percent of the population – currently suffer from cardiovascular disease (CVD). The term cardiovascular disease covers a broad spectrum of disorders that include high blood pressure, coronary heart disease (heart attack and chest pain), stroke, birth defects of the heart and blood vessels, and congestive heart failure.
 Every year heart attacks and stroke cause more than 930,000 deaths in the United States, making CVD the leading cause of death and accounting for 40 percent of deaths from all causes. And
Every year heart attacks and stroke cause more than 930,000 deaths in the United States, making CVD the leading cause of death and accounting for 40 percent of deaths from all causes. And
while CVD primarily kills people 65 and older, incidence of sudden deaths from heart disease is rising in people aged 15 to 34. (1)
Reducing serum cholesterol levels – especially the low-density (LDL) fraction – is a well-established, effective strategy for preventing cardiovascular disease and reducing coronary events and mortality. (2,3) Unfortunately a recent report in the journal Circulation found that between 1988 and 2000 average total serum cholesterol concentrations in the US population declined by a mere 1%. (4) And while 91% of respondents to a survey conducted by the American Heart Association felt it was “important to them personally to have a healthy cholesterol level,” fewer than 50% knew their own cholesterol level, and 53% either didn’t know, or overestimated, the recommended cholesterol level for a healthy adult. (5)
Compounding the problem, only a fraction of those at risk of cardiovascular disease are utilizing pharmaceutical and nutritional strategies known to reduce cholesterol levels. According to estimates based on data gathered from the National Health and Nutrition Examination Survey III (NHANES III), only 1.4 million (6.6%) of 21.1 million Americans eligible for cholesterol-lowering drug therapy under National Cholesterol Education Program (NCEP) guidelines were actually using such therapy. (6) And when researchers examined responses gathered from 13,990 patients they discovered that fewer than 4% of those diagnosed with hypercholesterolemia (elevated cholesterol) were taking vitamins or supplements known to reduce cholesterol. (7)
Concerned with the persistent failure of conventional strategies to significantly improve cholesterol profiles and reduce incidence of cardiovascular diseases, a broad coalition of medical researchers and scientists are now calling for a massive increase in the use of cholesterol-lowering drugs, particularly the family of pharmaceuticals known as statins. (8) Unfortunately statin drugs, while very effective, also have a number of serious side effects that understandably compromise patient compliance. Additionally, statin drugs are expensive to use – depending on the drug and the dosage, statin therapy can cost between $63-$228 per month. (9)
Now a newly available, all-natural supplement has been shown in human studies to significantly lower cholesterol levels – particularly LDL cholesterol, triglycerides, and ApoB – to aid in reducing ones risk of developing cardiovascular disease. The supplement, Sytrinol, is an important option for health-conscious consumers seeking a safe, effective and convenient solution for lowering cholesterol levels without the side effects and expense of pharmaceutical drugs.
Cholesterol and Human Health
Cholesterol is a fatty (lipid) component found in virtually all cell membranes. In addition to supporting cellular integrity, cholesterol is also required for the transport of phospholipids and the biosynthesis of mineralocorticoids (aldosterone), glucocorticoids (cortisol) and sex hormones (progesterone, pregnenolone, testosterone, estradiol). Far from endangering health, cholesterol is essential to life. In fact, researchers in Italy have shown that when serum cholesterol levels are too low (less than 160 mg/dL) mortality in older adults actually increases. (10,11)
In the body cholesterol is transported by two specialized carrier proteins – low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL, the “bad cholesterol,” is the primary carrier of cholesterol in the blood. In atherosclerosis, LDL is taken up in lesions in endothelial cells lining the inner walls of blood vessels, forming deposits in the arterial walls. Next the deposited LDL undergoes modification as free radicals oxidize LDL to form foam cells that form a thick, hard plaque.
Over time plaque accumulation can constrict vessels, inhibiting blood flow and reducing the supply of oxygen reaching the heart, brain and other organs. (12) If a clot (thrombus) blocks an artery already restricted by plaque, the flow of blood and oxygen can be cut off entirely, leading to a heart attack (if the occlusion occurs in the heart), or a stroke (if it occurs in the brain).
HDL cholesterol is referred to as “good” cholesterol because of its ability to aid in removing excess cholesterol from atherosclerotic deposits and retarding the growth of new plaque. In contrast to high LDL cholesterol levels, low HDL cholesterol levels have been shown to be an additional risk factor for increased mortality from coronary artery disease and strokes in the elderly. (13).
How the Body Manages Cholesterol Levels
While cholesterol levels can be modestly influenced by dietary modification (i.e.. reducing one’s intake of saturated and trans-fatty acids), (14) the majority of cholesterol (about 80%) doesn’t come from dietary sources, but is synthesized by the liver. Biosynthesis of cholesterol is controlled by the rate-limiting enzyme, HMG-CoA (3-hydroxy-3-methylglutaryl coenzyme A) reductase.
 Normally the liver regulates cholesterol levels via a biochemical feedback loop. When cholesterol levels are low, liver production of HMG-CoA reductase increases to speed up biosynthesis of cholesterol. Conversely, when cholesterol levels are too high, the liver limits HMG-CoA reductase production of to reduce the production of cholesterol. The proper functioning of this feedback mechanism is vital for the maintenance of healthy cholesterol levels. Unfortunately modern dietary habits and lifestyle contribute to the disruption of this system, leading to elevated cholesterol levels and increased risks for developing CVD.
Normally the liver regulates cholesterol levels via a biochemical feedback loop. When cholesterol levels are low, liver production of HMG-CoA reductase increases to speed up biosynthesis of cholesterol. Conversely, when cholesterol levels are too high, the liver limits HMG-CoA reductase production of to reduce the production of cholesterol. The proper functioning of this feedback mechanism is vital for the maintenance of healthy cholesterol levels. Unfortunately modern dietary habits and lifestyle contribute to the disruption of this system, leading to elevated cholesterol levels and increased risks for developing CVD.
Additionally, certain genetic disorders, such as familial hypercholesterolemia and autosomal recessive hypercholesterolemia, are known to increase LDL levels and risk of developing CVD. (15)
Not All LDL is Created Equal
In order to bind with other molecules for transport through the circulatory system lipids rely on a specialized class of structural proteins, called apoproteins. Low-density lipoprotein (LDL) exists in two versions, differentiated by their protein components. The first, apo-A, is bound to a large, “fluffy” protein called apolipoprotein A (apo-A) that has been shown to protect against heart disease. The second, apo-B, is bound to a small, very dense protein called apolipoprotein B that plays a major role in cardiovascular disease. Apo-B particles enable cholesterol to penetrate and lodge in vascular walls, a key step in initiating the formation of atherosclerotic plaque. (16) Apo-B is the predominant form of apolipoprotein, and over 90% of all LDL cholesterol particles in the blood carry apo-B, making it an especially accurate (and convenient) marker for measuring the cholesterol-depositing capacity of blood. (17-19)
The importance of apo(B) was highlighted in a report on the Apoliprotein-Related Mortality Risk Study (AMORIS), published in the Dec. 15, 2001 issue of Lancet. In the AMORIS study, researchers evaluated cardiovascular markers in over 175,000 men and women over a period of 51/2-years. In addition to conventional lipid markers, such as triglycerides, total cholesterol, and LDL-HDL ratios, the researchers also measured apo(B) levels. Their findings revealed that those persons with the highest ratios of apo(B) to apo(A) were at the greatest absolute risk of dying from a heart attack. (20).
These findings were supported by a second study, published in the Nov. 11, 2003 issue of the journal, Circulation. In the Insulin Resistance Atherosclerosis Study (IRAS) researchers again measured apoB levels in 1522 individuals and compared them with an array of standard lipid markers (C-reactive protein, fibrinogen, plasminogen activator inhibitor-1 [PAI-1], fasting and post-glucose load glucose and insulin concentrations, and carotid artery intima-media thickness) to assess CVD risks. They found that elevated apo-B levels were strongly associated with CVD, and concluded that apo-B levels are a better predictor of vascular risk than LDL cholesterol. (21)
Given the well-documented link between apo-B and cardiovascular disease, measuring apo-B levels offers clinicians and patients a new and highly specific marker for assessing both the precise level of LDL in serum and determining individual risk for developing CVD.
Statins Drugs: the New Aspirin?
Due to the failure of previous public health programs to substantially lower cholesterol levels in the general population, medical researchers and health experts are seeking a new approach to better manage the problem. For the last decade physicians and patients have relied on cholesterol guidelines published by the American Heart Association (AHA). According to the AHA, total cholesterol (TC) levels of 200 mg/dL or less are considered to be optimal. Levels between 200 mg/dL and 239 mg/dL are considered borderline high risk, and anything above 240 mg/dL is considered high risk.
In May, 2001, the National Institutes of Health (NIH) published new federal guidelines, Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), that called for an aggressive expansion in the use of statin drugs to treat cholesterol. (22) Statin drugs, such as atorvastatin (Lipitor), lovastatin (Mevacor®), pravastatin (Pravachol) and simvastatin (Zocor), are among the most potent lipid-lowering agents currently available.
Statins lower cholesterol levels by inhibiting the production HMG-CoA reductase, resulting in a decrease in cholesterol synthesis in the liver. To compensate for the resulting reduction of cholesterol production the liver begins to remove LDL cholesterol circulating in the blood, further reducing overall LDL cholesterol levels. Statin therapy has been proven to contribute to a substantial decrease in CHD morbidity and mortality in recent years, as documented in a number of controlled clinical trials. (23) In addition to improvements in lipid profile, statins also appear to confer other benefits, including improved endothelial function, decreased platelet thrombus formation, improved fibrinolytic activity and reduction in frequency of transient myocardial ischemia. (24)
Although statin therapy was initially used to treat patients suffering from severe hypercholesterolemia, health experts have recently begun to push for a dramatic increase the use of statin drugs to aggressively drive down cholesterol levels in patients with only moderately elevated cholesterol. A growing body of health experts has called for more widespread use of statins to include not just those with high cholesterol, but also those with diabetes, high blood pressure, high serum triglycerides, low HDL, and those with a strong family history of heart disease.
Most recently, the July 13 issue of Circulation published an updated version of the National Cholesterol Education Program (NCEP) encouraging physicians to aggressively increase the use of statin drugs to lower cholesterol levels. In particular, the report recommends that target LDL levels be reduced from the current 100 mg/dL down to 70 mg/dL in patients considered at high risk for a heart attack or death from CVD. Additionally, patients only at moderate risk of a heart attack – those with heart disease, diabetes or other risk factors – are now being encouraged to reduce their cholesterol levels by 30 to 40%. (25)
Not surprisingly, the new guidelines could ultimately increase the number of patients on statin drugs to as many as 50 million users. (26) However, in an embarrassing oversight, the same government panel drafting the new guidelines failed to mention in the report that several of the panelists are linked to some of the pharmaceutical companies that manufacture statin drugs. In fact, six of the nine panelists had either received grants or were paid consulting or speakers’ fees by the companies that produce some of the most popular statin medications on the market, including: Pfizer’s Lipitor; Bristol-Myers Squibb’s Pravachol; Merck’s Lovastatin; and AstraZeneca’s Crestor. (27)
Statins and Side Effects
While statin drugs are effective at lowering LDL cholesterol, they also present a number of serious side effects. In 1990, Folkers theorized that inhibition of HMG-CoA reductase would also inhibit intrinsic biosynthesis of coenzyme Q10 (CoQ10), a central compound in the mitochondrial respiratory chain. The researchers stated “If lovastatin were to reduce levels of CoQ10, this reduction would constitute a new risk of cardiac disease, since it is established that CoQ10 is indispensable for cardiac function.”
When the researchers examined five hospitalized patients, aged 43 to 72, they found that lovastatin in fact did cause CoQ10 levels to drop. Furthermore, the patients showed evidence of increased cardiac distress, a potentially a life-threatening situation for patients hospitalized with class IV cardiomyopathy. The researchers concluded that, “Although a successful drug, lovastatin does have side effects, particularly including liver dysfunction, which presumably can be caused by the lovastatin-induced deficiency of CoQ10.”(28) And while taking supplemental CoQ10 may potentially offset this side effect, other, much more serious side effects cannot be so easily resolved.
For example, rhabdomyolysis is a rare but potentially deadly condition that occurs when large numbers of skeletal muscle cells die. As the rapidly dying cells deteriorate they release large quantities of muscle proteins into the bloodstream, quickly overwhelming the kidneys. An analysis of the Food and Drug Administration’s side-effect registry, conducted in 2001 by the consumer watchdog group Public Citizen, discovered that statin drugs were linked to 72 fatal, and 772 non-fatal cases of rhabdomyolysis between October 1997 and December 2000. Following the report, in August, 2001, pharmaceutical giant Bayer AG was forced to remove its statin drug, Baycol (cerivastatin), from the market after it was found to be responsible for killing at least 31 people. (29)
More recently, an article in the June 26 2004 issue of The Lancet, raises issue with the FDA for approving one of the newest statin drugs, Crestor, despite pre-approval evidence that the drug caused rhabdomyolysis. According to the author, Dr. Sidney Wolfe, director of Public Citizen’s Health Research Group, Crestor was approved despite an FDA claim that new cholesterol drugs would only be approved only if they presented a comparable or lower risk of rhabdomyolysis than drugs already on the market.
According to Wolfe, patients taking Crestor experienced severe muscle deterioration at higher rates than patients taking other cholesterol-lowering drugs. In fact, the rate of post-marketing reports of rhabdomyolysis for Crestor appears to exceed that of all other currently marketed statins. From its approval in August 2003 to mid-April, 18 patients, including 11 in the United States, suffered severe muscle deterioration. In addition, eight cases of acute kidney failure and four cases of kidney insufficiency related to the use of Crestor have been reported. (30)
Unknown Long-Term Effects
While the cardio-protective benefits of statin drugs outweigh the known side effects, the most recent NCEP recommendations may result in tens of millions of new patients taking statins for a period of decades, and possibly for a lifetime. Unfortunately data on long-term use of statins is scant. In one paper published in the Journal of the American Medical Association in 1996, researchers set off a furious round of debate by raising the possibility of long-term statin use causing cancers. In the original paper, authors Newman and Hulley pointed out that all statin drugs have been shown to induce cancer in experimental lab rodents, and in some cases the amount of statins causing cancer in animals matched dosages being prescribed to humans. While conceding that extrapolating incidence of cancer in rodents to humans is “an uncertain process,” the authors recommended that “lipid-lowering drug treatment, especially with the fibrates and statins, should be avoided except in patients at high short-term risk of coronary heart disease.” (31)
Another potentially serious long-term problem appeared in a case study initiated after several reports and a single epidemiologic study suggested that statins cause damage to the peripheral nervous system. In the 2002 paper, after reviewing patient records from 1994 to 1998, the authors verified diagnosis of idiopathic polyneuropathy in 166 patients receiving statin therapy for at least two years, concluding that “long-term exposure to statins may substantially increase the risk of polyneuropathy.” (32)
Healthy Options for Lowering Cholesterol
In their enthusiasm to reduce premature deaths from heart attack and strokes the authors of the new cholesterol guidelines are recommending that millions of Americans be put on statin drugs while ignoring warning signs of potentially serious side effects from the long-term use of these compounds. Would informed health consumers willingly choose to lower their risk of CVD if it meant substantially increasing their chances for developing cancer or ALS after a decade or two?
In a recent op-ed piece in the Washington Post, Dean Ornish, clinical professor of medicine at the University of California, San Francisco and president of the nonprofit Preventive Medicine Research Institute, pointed out that “As tens of millions of people begin taking these medications for decades, more long-term side effects are likely to become apparent.” Ornish also questioned why the panel failed to recommend other options, such as diet and lifestyle changes that, for most people, “can be a safe and effective alternative to a lifetime of cholesterol-lowering drugs?” (33)
One of the newest and most effective alternatives to statin drugs is a recently patented proprietary formula comprising citrus and palm fruit extracts that contains polymethoxylated flavones and tocotrienols. It has been shown in human trials to significantly reduce total cholesterol, LDL cholesterol, and triglycerides. Additionally, the powerful antioxidant and anti-inflammatory properties of the extracts in this nantural formulation (trademarked under the name Sytrinol™) are known to contribute to managing additional cardiovascular disease risk factors.
Tangerine Flavonoids Safer than Statins
Flavonoids are natural polyphenolic compounds found in a wide variety of fruits and vegetables. Over 4,000 different flavonoids have been identified, and many of these have been shown to exert biological effects in humans. Bioflavonoids from citrus fruits, such as oranges, tangerines and grapefruits have been found to reduce oxidative drug metabolism, (34-36) inhibit chemical carcinogenesis and tumor development (37-39) and exert anti-inflammatory and antiallergic effects (40) Additionally, epidemiological studies have shown that intake of dietary flavonoids is strongly associated with reduced incidence of cardiovascular disease and cancer in humans. (41)
Many flavonoids, such as rutin and hesperidin, exert cardiovascular protection by inhibiting oxidation of LDL cholesterol, reducing inflammation, enhancing endothelial function, and reducing thrombosis. (42,43) Recently a subset of flavonoids known as polymethoxylated flavones (PMFs), have been shown to possess especially potent anti-cancer, immuno-supportive and cardio-protective benefits. Polymethoxylated flavones are flavonoid compounds derived from the peels of oranges, tangerines and other citrus fruits. PMFs are highly methoxylated, meaning that one or more of the -H groups are replaced by CH2O. This natural process results in a more biologically active molecule with distinctly unique metabolic properties. Two of the most well-researched PMFs, are the flavonoids nobiletin and tangeretin.
Nobiletin was first isolated from orange peels in 1938. (44) Intrigued with the anti-cancer benefits associated with citrus fruit consumption, researchers first examined nobiletin as a potential chemopreventive compound. (45). Early studies revealed that nobiletin significantly inhibits production of nitric oxide (NO) and superoxide (O2-), two powerful free radicals involved in promoting inflammation and cancer. In one study nobiletin was shown to suppress several stages of skin inflammation required for tumor initiation and growth. (46) Nobiletin has also been shown to inhibit the proliferation of human gastric cancer cells (metastases) in mice, leading study authors to suggest that the compound “may be a candidate anti-metastatic drug for prevention of peritoneal dissemination of gastric cancer.” (47)
Nobiletin has also been shown to be a powerful anti-inflammatory agent. Atherosclerosis is now recognized to be an inflammatory process, partially explaining why half of all heart attacks occur in people with “normal” cholesterol levels. In one study, patients were monitored after being diagnosed with unstable angina. After one year, sixty-nine percent of the patients with elevated CRP had experienced a heart attack, compared to significantly fewer heart attacks and increased survival in patients with lower CRP levels less. (48)
In addition to serving as a significant marker of vascular inflammation and a risk factor for cardiovascular disease and heart attacks, C-reactive protein also has been shown to actively damage blood vessel walls by blocking a critical protector protein and promoting plaque formation. Researchers from UC Davis found that C-reactive protein inhibits the activity of a critical ‘protector’ enzyme – eNOS or endothelial nitric oxide synthase – that prevents heart disease by inhibiting plaque from adhering to blood vessel walls, keeping coronary arteries dilated and inhibiting constriction of smooth muscle cells. (49)
While popular nonsteroidal anti-inflammatory drugs, such as Celebrex and Vioxx, reduce inflammation by blocking the enzyme cyclooxygenase-2 (COX-2), these drugs have recently come under scrutiny for possibly increasing the risk of cardiovascular events. By contrast, nobiletin has been found to selectively downregulate COX-2 without interfering with COX-1 mRNA expression. (50) Nobiletin was also shown to suppress production of prostaglandin (PG) E(2), while interfering with pro-inflammatory cytokines such as interleukin-1alpha, interleukin-1beta, TNF-alpha and interleukin-6, in mouse macrophages. (51) These anti-inflammatory effects, which are comparable to those of such powerful anti-inflammatory steroids as dexamethasone, support the characterization of nobiletin as a powerful anti-inflammatory compound.
In addition, nobiletin has demonstrated greater anti-inflammatory activity than indomethacin in a TPA-induced edema test in mouse ears, further supporting the anti-oxidative, anti-inflammatory, and cancer preventive benefits of nobiletin. (52)
Tangeretin is a polymethoxylated flavone (PMF), first isolated from tangerine oil in 1934. (53) Early research found that tangeretin has anti-cancer actions similar to those of similar PMFs, such as nobiletin and quercetin. (54-56) Tangeretin was also found to exert antioxidant and neuroprotective benefits. In an animal study tangeretin was found to cross the blood-brain barrier and protect brain cells (hypothalamus, striatum and hippocampus) in rats exposed to 6-hydroxydopamine (6-OHDA). 6-OHDA is a drug that produces cytolytic free-radicals that deplete noradrenergic stores in nerve endings and reduce dopamine levels in the brain (thus providing a model of Parkinson’s disease.) (57)
Recently, in vitro studies have revealed that tangeretin lowers triglyceride and apo-B levels. (58) Researchers in Canada first observed that intracellular production of cholesterol and apo-B declined rapidly in human liver cells after being incubated with tangeretin. When they followed up on their initial findings the researchers determined that tangeretin achieves these reductions by modulating several mechanisms involved in lipoprotein metabolism.
First tangeretin was shown to interfere with cellular production of triglycerides (TG), reducing levels up to 37% following treatment. Tangeretin was also found to reduce intracellular production of apo-B by inhibiting microsomal triglyceride transfer protein (MTP), a specialized lipid transfer protein with a key role in the assembly and secretion of lipoproteins containing apo-B. By limiting MTP, tangeretin reduces the number of apo-B particles that can be synthesized in the liver. (59)
Additionally, the researchers discovered that tangeretin helps to limit apo-B production by suppressing diacylglycerol acyltransferase (DGAT), the final enzyme in the pathway of TG synthesis. Triglycerides play an important role in the formation of apo-B. By limiting production of TG tangeretin effectively restricts production of apo-B. (60)
Tocotrienols – A Natural Alternative to Statin Drugs
Tocotrienols are a third cardio-protective component of the proprietary Sytrinol formula. Tocotrienols are naturally occurring antioxidant analogs of tocopherols (natural vitamin E). Most risk markers for cardiovascular disease have a pro-inflammatory component, which stimulates the release of a number of active molecules (such as inflammatory mediators, reactive oxygen species, nitric oxide, and peroxynitrite from endothelial, vascular smooth muscle, and immune cells) in response to injury. In addition to its antioxidant effects, tocotrienols have been shown to exert a powerful anti-inflammatory effect that can help to mitigate inflammatory processes that are known to initiate atherosclerosis. (61)
Tocotrienols have also been found to lower total serum cholesterol and LDL-cholesterol levels by degrading the enzyme, HMG-CoA-reductase, responsible for producing cholesterol. By inhibiting the enzymatic actions of HMG-Co-A reductase through a post-transcriptional mechanism, tocotrienols can suppress cholesterol synthesis without the harmful side effects observed with statin drugs. (62)
Early research found that tocotrienols from palm oil sources reduced cholesterol, LDL and triglycerides, while raising high-density lipoprotein (HDL) levels. An observed secondary benefit from tocotrienols was an increase in apo-A levels, which counter the damaging effects of apo-B. (63)
In animal studies tocotrienols were shown to reduce total cholesterol by 30%, and LDL by 67% compared to controls in rats with induced hypercholesterolemia. Tocotrienols were also shown to significantly reduce HMG-CoA activity. (64)
In a 2002 study of humans, 90 subjects diagnosed with hypercholesterolemia were treated with a protocol that utilized tocotrienols in conjunction with the American Heart Association (AHA) Step-1 diet. The researchers reported that treatment with 100 mg/day of a tocotrienol-rich supplement resulted in a 20% decrease of total cholesterol, 25% decrease in LDL, 14% decrease in apo-B, and a 12% drop in triglycerides. (65)
Safety and Effects of Sytrinol™
Sytrinol™ was developed after 12 years of extensive research on polymethoxylated flavonoids, tocotrienols, and their effects on cardiovascular health. The health benefits of Sytrinol have been well demonstrated in in vitro, in vivo, and clinical studies. Animal toxicity studies have shown that Sytrinol is well tolerated, with no toxics effects following consumption of polymethoxylated flavones in amounts up to 1% of the diet – the equivalent of an 70 kg (150 lbs.) individual consuming almost 14 grams per day.
The cholesterol-lowering effects of Sytrinol were documented in a recent animal study published in the May 2004 issue of the Journal of Agricultural and Food Chemistry. Canadian researchers first induced hypercholesterolemia in hamsters to boost their cholesterol levels. The animals were then treated with either PMFs (tangeretin) or a combination of flavones (hesperidin and naringin). While the flavones were shown to lower cholesterol levels, the PMF formulation proved to be almost three times as effective. In hamsters receiving the tangeretin formula, total cholesterol levels declined by up to 27%, and LDL was reduced by 40%. And while HDL levels were unchanged, the net result was a significant improvement in the LDL/HDL ratio. (66)
The cardio-protective and cholesterol-lowering claims for Sytrinol™ are also supported by human trials. Two early trials, consisting of ten subjects each, measured the effects of Sytrinol in men and women diagnosed with hypercholesterolemia (elevated cholesterol) and screened to eliminate thyroid disorders, kidney disorders and diabetes. Subjects were instructed to maintain normal dietary habits and discontinue using vitamins, supplements and cholesterol-lowering medications for at least 6 weeks prior to, and during the study. Fasting blood samples were drawn at the onset and end of each 4-week trial, and plasma lipids profiles and other metabolic parameters were analyzed using standard methods.
The results from the first trial (Table 1) show that four weeks of treatment with Sytrinol (300 mg/day) caused significant reductions in: total cholesterol (-24%); LDL cholesterol (-19%); and triglycerides (-23%). There were no changes in HDL cholesterol levels, and body mass remained relatively stable.
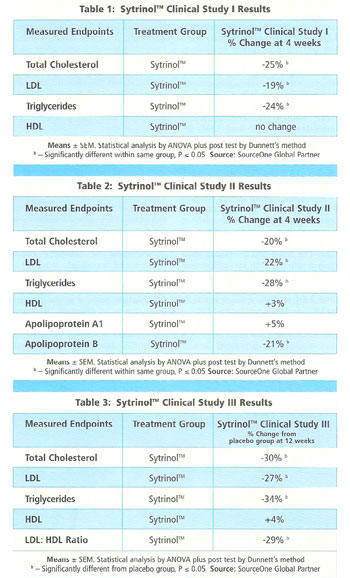
In the second trial, subjects with elevated cholesterol again benefited from only four weeks of treatment with Sytrinol (300 mg/day). As Table 2 illustrates, treatment with Sytrinol caused significant reductions in: plasma total cholesterol (19.7%); LDL cholesterol (22.01%); apo B (20.9%); and triglycerides (28.4%). Additionally, subjects in the second trial benefited from a significant 5% increase in apo-A1, an important structural protein of HDL
Sytrinol is currently being tested in a long-term, double-blind, crossover randomized study involving 120 men and women with moderately elevated cholesterol levels (total cholesterol >230 mg/dL, LDL > 155 mg/dL). Subjects will receive either 300 mg/day of Sytrinol or a placebo for 12 weeks, followed by a washout period (4 weeks), followed by another 12 weeks where the groups receiving the active compound or placebo will be crossed over.
Only the first 12 weeks (Phase 1) of the long-term study have been completed, yet already the results are proving to be compelling. Compared to placebo, total cholesterol was reduced 30%, LDL cholesterol was reduced 27% and total triglycerides dropped 33%. Additionally, HDL cholesterol levels increased by 4%, resulting in a significant reduction in the LDL/HDL ratio of 30%.
Conclusion
Cholesterol management is a well-established means of maintaining health and preventing premature death from cardiovascular disease. Many people can maintain desirable cholesterol profiles by natural means, including lifestyle modification, exercise, dietary strategies and natural hormone replacement protocols. For those in need of additional cholesterol-lowering options, Sytrinol is a new and important option that can aid in achieving substantial reductions in total cholesterol, LDL cholesterol and triglyceride levels, while improving LDL/HDL ratios. Its lack of the side effects associated with statin drugs makes Sytrinol™ an especially attractive therapy for maintaining healthy cholesterol levels.
References
1. Preventing Heart Disease and Stroke. Addressing the Nation’s Leading Killers At A Glance 2004. National Center for Chronic Disease Prevention and Health Promotion. http://www.cdc.gov/nccdphp/aag/aag_cvd.htm).
2. Wald NJ, Law MR. Serum cholesterol and ischaemic heart disease. Atherosclerosis. 1995 Dec;118 Suppl:S1-5.
3. Barter P. Treatment of dyslipidaemia in high-risk patients: too little, too late. Int J Clin Pract Suppl. 2002 Jul;(130):15-9.
4. Ford ES, Mokdad AH, Giles WH, Mensah GA. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation. 2003 May 6;107(17):2185-9.
5. Nash IS, Mosca L, Blumenthal RS, Davidson MH, Smith SC Jr, Pasternak RC. Contemporary awareness and understanding of cholesterol as a risk factor: results of an American Heart Association national survey. Arch Intern Med. 2003 Jul 14;163(13):1597-600.
6. Gotto AM Jr. Lipid management in patients at moderate risk for coronary heart disease: insights from the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Am J Med. 1999 Aug 23;107(2A):36S-39S.
7. Steyer TE, King DE, Mainous AG 3rd, Gilbert G. Use of nutritional supplements for the prevention and treatment of hypercholesterolemia. Nutrition. 2003 May;19(5):415-8.
8. Ref for calls to increase statin drug use
9. Perreault S, Hamilton VH, Lavoie F, Grover S. Treating hyperlipidemia for the primary prevention of coronary disease. Are higher doses of lovastatin cost-effective? Arch Intern Med 1998;158:375-381.)
10. Onder G, Landi F, Volpato S, Fellin R, Carbonin P, Gambassi G, Bernabei R. Serum cholesterol levels and in-hospital mortality in the elderly. Am J Med. 2003 Sep;115(4):265-71.
11. Brescianini S, Maggi S, Farchi G, Mariotti S, Di Carlo A, Baldereschi M, Inzitari D; ILSA Group. Low total cholesterol and increased risk of dying: are low levels clinical warning signs in the elderly? Results from the Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2003 Jul;51(7):991-6.
12. Brown MD, Jin L, Jien ML, Matsumoto AH, Helm GA, Lusis AJ, Frank JS, Shi W. Lipid retention in the arterial wall of two mouse strains with different atherosclerosis susceptibility. J Lipid Res. 2004 Jun;45(6):1155-61. Epub 2004 Mar 16.
13. Weverling-Rijnsburger AW, Jonkers IJ, van Exel E, Gussekloo J, Westendorp RG. High-density vs low-density lipoprotein cholesterol as the risk factor for coronary artery disease and stroke in old age. Arch Intern Med. 2003 Jul 14;163(13):1549-54.
14. Wald NJ, Law MR. Serum cholesterol and ischaemic heart disease. Atherosclerosis. 1995 Dec;118 Suppl:S1-5.
15. Pullinger CR, Kane JP, Malloy MJ. Primary hypercholesterolemia: genetic causes and treatment of five monogenic disorders. Expert Rev Cardiovasc Ther. 2003 May;1(1):107-19.
16. Gustafsson M, Flood C, Jirholt P, Boren J. Retention of atherogenic lipoproteins in atherogenesis. Cell Mol Life Sci. 2004 Jan;61(1):4-9.
17.Cabezas Castro M, Liem A. The use of apolipoprotein B in clinical practice to determine the risk for atherosclerosis. Ned Tijdschr Geneeskd. 2003 Jul 26;147(30):1445-8.
18, Walldius G, Jungner I. Apolipoproteins are new and better risk indicators of myocardial infarction. Lakartidningen. 2004 Mar 25;101(13):1188-94.
19.Walldius G, Jungner I. Apolipoprotein B and apolipoprotein A-I: risk indicators of coronary heart disease and targets for lipid-modifying therapy. J Intern Med. 2004 Feb;255(2):188-205.
20, Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001 Dec 15;358(9298):2026-33
21. Williams K, Sniderman AD, Sattar N, D’Agostino R Jr, Wagenknecht LE, Haffner SM. Comparison of the associations of apolipoprotein B and low-density lipoprotein cholesterol with other cardiovascular risk factors in the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2003 Nov 11;108(19):2312-6. Epub 2003 Oct 27.
22. National Health and Nutrition Examination Study III (NHANES III, 1988–94) (CDC) NCHS.
23. Farmer JA. Aggressive lipid therapy in the statin era. Prog Cardiovasc Dis 1998;41:71-94.
24. Farnier M, Davignon J. Current and future treatment of hyperlipidemia: the role of statins. Am J Cardiol 1998 Aug 27 82:4B 3J-10J
25. Grundy SM, Cleeman JI, Bairey Merz CN, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ; for the Coordinating Committee of the National Cholesterol Education Program. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004; 110:227-239.
2. Matthew Herper. Cholesterol Guidelines A Gift For Merck, Pfizer. 07.12.04, 4:30 PM ET Forbes Magazine
27. Delthia Ricks, Roni Rabin, Panel’s ties to drugmakers not cited in new cholesterol guidelines. Newsday.com
28, Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, Tamagawa H. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci U S A 1990 Nov 87:22 8931-4.
29. Bayer Voluntarily Withdraws Baycol. FDA Talk Papers T01-34, August 8, 2001.
30. Sidney M Wolfe. Dangers of rosuvastatin identified before and after FDA approval. The Lancet, Volume 363, Issue 9427, 26 June 2004,Pages 2189-2190.
1. Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA. 1996 Jan 3;275(1):55-60.
32. D. Gaist, MD PhD, U. Jeppesen, MD PhD, M. Andersen, MD PhD, L. A. García Rodríguez, MD MSc, J. Hallas, MD PhD and S. H. Sindrup, MD PhD. Statins and risk of polyneuropathy: A case-control study. Neurology 2002;58:1333-1337.
33.Dean Ornish. Lower Cholesterol Without Drugs. The Washington Post. Sunday, August 8, 2004; Page B07.
34. Buening MK, Chang RL, Huang M-T, Fortner JG, Wood AW and Conney AH (1981) Activation and inhibition of benzo(a)pyrene and aflatoxin B1 metabolism in human liver microsomes by naturally occurring flavonoid. Cancer Research, Vol 41, Issue 1 67-72.
35.Siess M-H, Guillermic M, Le Bon AM and Suschetet M (1989) Induction of monooxygenase and transferase activities in rat by dietary administration of flavonoids. Xenobiotica. 1989 Dec;19(12):1379-86.
36. Guengerich FP and Kim DH (1990) In vitro inhibition of dihydropyridine oxidation and aflatoxin B1 activation in human liver microsomes by naringenin and other flavonoids. Carcinogenesis, Vol 11, 2275-2279.
37.Mukhtar H, Das M, Khan WA, Wang ZY, Bik DP and Bickers DR (1988) Exceptional activity of tannic acid among naturally occurring plant phenols in protecting against 7,12-dimethylbenz(a) anthracene-, benzo(a)pyrene-, 3-methylcholanthrene-, and N-methyl-N-nitrosourea-induced skin tumorigenesis in mice. Cancer Research, Vol 48, Issue 9 2361-2365.
38.Verma AK, Johnson JA, Gould MN and Tanner MA (1988) Inhibition of 7,12-dimethylbenz(a)anthracene and N-nitrosomethylurea induced rat mammary cancer by dietary flavonol quercetin. Cancer Research, Vol 48, Issue 20 5754-5758.
39.Firenzuoli F, Gori L, Crupi A, Neri D. Flavonoids: risks or therapeutic opportunities? Recenti Prog Med. 2004 Jul-Aug;95(7-8):345-51.
40. Middleton E and Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem Pharmacol. 1992 Mar 17;43(6):1167-79..
41.Maron DJ. Flavonoids for reduction of atherosclerotic risk. Curr Atheroscler Rep. 2004 Jan;6(1):73-8.
42.Milde J, Elstner EF, Grassmann J. Synergistic inhibition of low-density lipoprotein oxidation by rutin, gamma-terpinene, and ascorbic acid. Phytomedicine. 2004 Feb;11(2-3):105-13.)
43.Monforte MT, Trovato A, Kirjavainen S, Forestieri AM, Galati EM, Lo Curto RB. Biological effects of hesperidin, a Citrus flavonoid. (note II): hypolipidemic activity on experimental hypercholesterolemia in rat. Farmaco. 1995 Sep;50(9):595-9.
44.Tseng KF (1938) Nobiletin. Part I., an oil extracted by cold methyl alcohol from Citrus nobilis, Lour, affords nobiletin, a hexamethoxyflavone containing a veratryl nucleus. Chem Soc 1003-1004.
45.Manthey JA, Grohmann K, Guthrie N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 2001 Feb;8(2):135-53.
46.Murakami A, Nakamura Y, Torikai K, Tanaka T, Koshiba T, Koshimizu K, Kuwahara S, Takahashi Y, Ogawa K, Yano M, Tokuda H, Nishino H, Mimaki Y, Sashida Y, Kitanaka S, Ohigashi H. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000 Sep 15;60(18):5059-66.
47.Minagawa A, Otani Y, Kubota T, Wada N, Furukawa T, Kumai K, Kameyama K, Okada Y, Fujii M, Yano M, Sato T, Ito A, Kitajima M. The citrus flavonoid, nobiletin, inhibits peritoneal dissemination of human gastric carcinoma in SCID mice. Jpn J Cancer Res. 2001 Dec;92(12):1322-8.
48. Kushner, I. C-reactive protein elevation can be caused by conditions other than inflammation and may reflect biologic aging. Cleveland Clinic Journal of Medicine, 2001, 68: 6, 535-540.
49.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002 Sep 17;106(12):1439-41.
50. O’Leary KA, de Pascual-Tereasa S, Needs PW, Bao YP, O’Brien NM, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat Res. 2004 Jul 13;551(1-2):245-54.
51.Lin N, Sato T, Takayama Y, Mimaki Y, Sashida Y, Yano M, Ito A. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem Pharmacol. 2003 Jun 15;65(12):2065-71.
52.Murakami A, Nakamura Y, Ohto Y, Yano M, Koshiba T, Koshimizu K, Tokuda H, Nishino H, Ohigashi H. Suppressive effects of citrus fruits on free radical generation and nobiletin, an anti-inflammatory polymethoxyflavonoid. Biofactors. 2000;12(1-4):187-92.
53.Nelson EK (1934) The occurrence of a pentamethyl flavonol in tangerine peel. J Am Chem Soc 56 :1392.
54.Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Effect of citrus flavonoids on HL-60 cell differentiation. Anticancer Res. 1999 Mar-Apr;19(2A):1261-9.
55.Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M. Antiproliferative activity of flavonoids on several cancer cell lines. Biosci Biotechnol Biochem. 1999 May;63(5):896-9.
56.Manthey JA, Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J Agric Food Chem. 2002 Oct 9;50(21):5837-43.
57.Datla KP, Christidou M, Widmer WW, Rooprai HK, Dexter DT. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport. 2001 Dec 4;12(17):3871-5.
58.Kurowska EM, Manthey JA, Casaschi A, Theriault AG. Modulation of HepG2 cell net apolipoprotein B secretion by the citrus polymethoxyflavone, tangeretin. Lipids. 2004 Feb;39(2):143-51.
59.Gordon DA. Recent advances in elucidating the role of the microsomal triglyceride transfer protein in apolipoprotein B lipoprotein assembly. Curr Opin Lipidol. 1997 Jun;8(3):131-7.
60.Jamil H, Gordon DA, Eustice DC, Brooks CM, Dickson JK Jr, Chen Y, Ricci B, Chu CH, Harrity TW, Ciosek CP Jr, Biller SA, Gregg RE, Wetterau JR. An inhibitor of the microsomal triglyceride transfer protein inhibits apoB secretion from HepG2 cells. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11991-5.
61. Osiecki H. The role of chronic inflammation in cardiovascular disease and its regulation by nutrients. Altern Med Rev. 2004 Mar;9(1):32-53.
62. Sun W, Yan Y, Dong F. Progression of tocotrienols. Wei Sheng Yan Jiu. 2004 Mar;33(2):243-5.
63.Ong AS, Goh SH. Palm oil: a healthful and cost-effective dietary component. Food N63utr Bull. 2002 Mar;23(1):11-22.
64.Iqbal J, Minhajuddin M, Beg ZH. Suppression of 7,12-dimethylbenz[alpha]anthracene-induced carcinogenesis and hypercholesterolaemia in rats by tocotrienol-rich fraction isolated from rice bran oil. Eur J Cancer Prev. 2003 Dec;12(6):447-53.
65.Qureshi AA, Sami SA, Salser WA, Khan FA. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis. 2002 Mar;161(1):199-207.
66. Kurowska EM, Manthey JA. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. J Agric Food Chem. 2004 May 19;52(10):2879-86.
This article appeared in the November 2004 issue of Life Extension. Reprinted with permission of LE Publications, Inc.












[…] New Dietary Supplement Shows Dramatic Effects in LoweringNew Dietary Supplement Shows Dramatic Effects in Lowering Cholesterol, LDL, and Triglycerides. by tenglish on April 26, 2013 · 0 comments. in Nutrition … nutritionreview.org/2013/04/dietary-supplement-shows-dramatic-effects-lowering-c […]
“Sytrinol is currently being tested in a long-term, double-blind, crossover randomized study involving 120 men and women with moderately elevated cholesterol levels (total cholesterol >230 mg/dL, LDL > 155 mg/dL). Subjects will receive either 300 mg/day of Sytrinol or a placebo for 12 weeks, followed by a washout period (4 weeks), followed by another 12 weeks where the groups receiving the active compound or placebo will be crossed over.”
What is the citation information from this study. I would like to look it up. Is it available in Pub-Med or Clinical Trials database?