By Jim English
Rheumatoid arthritis (RA) is a chronic autoimmune disease that affects connective tissues and joints. Also known as atrophic and chronic proliferative arthritis, rheumatoid arthritis is marked by the interaction of pro-inflammatory and anti-inflammatory mechanisms that lead to inflammation of synovial membranes and contributes to bone erosion. While the specific cause of rheumatoid arthritis is unknown, genetic factors appear to play a role in the onset of the disease.
Western medicine utilizes several classes of drugs to treat Rheumatoid arthritis, including analgesics, corticosteroids, uric acid-lowering drugs, immunosuppressive drugs, non-steroidal anti-inflammatory drugs (NSAIDs), and disease-modifying anti-rheumatic drugs (DMARDs) such as antimalarial drugs, gold compounds, penicillamine and sulfasalazine. While many of these drugs provide temporary relief of pain and inflammation, they have little or no effect on the underlying disease processes. Long-term toxicity and side effects associated with approved RA drugs requires that patients be carefully monitored and frequently re-evaluated by their physicians.
Alternative nutritional approaches include joint-building substances like chondroitin sulfate, glucosamine, mucopolysaccharides, and MSM, and anti-inflammatory substances like proteolytic enzymes such as Wobenzyme, Serrapeptase, Boswellia Serrata Extract, Turmeric, and Essential Fatty Acids (EFAs).
A recently introduced addition to this armamentarium is a standardized extract derived from the root of the Peony plant. Peony has been found in clinical studies in China to alleviate symptoms associated with rheumatoid arthritis. Currently, this standardized extract is the treatment of choice for rheumatoid arthritis in China and other Asian countries. Peony extract has been proven to reduce pain, inflammation, and swelling, to support healthy joint function and to keep joints flexible and comfortable. Secondary benefits of the extract include memory improvement, normalization of blood sugar levels, and antispasmodic, sedative and analgesic effects.
History
Peony root is a major Chinese medicinal herb that has been used for thousands of years. Peony root was described in the Materia Medica of Traditional Chinese Medicine during the Han Dynasty (206 BC to 220 AD). Peony is also listed in over 30 formulas in a classic text of early Chinese medicine, the Shang Han Lun (Discussion of Cold-Induced Disorders), and is also listed in the pharmacopoeias of China and Japan. It is one of the commonly used ingredients in liver disease treatment. The primary active components of peony root are alkaloids, glucosides, polysaccharides, saponins, organic acids and trace minerals.
Total Glucosides of Peony (TGP)
In the mid-1980s researchers developed a process to extract a standardized compound containing a mini-mum of 40 percent paeoniflorin, the principal active ingredient in peony. This standardized extract, called Total Glucosides of Peony (TGP), has been the subject of extensive research in China for over 20 years. A number of clinical studies have found that TGP possesses both anti-inflammatory and immune modulation effects.
Animal Studies
In 1982 Japanese researchers first discovered that paeonin and paeoniflorin, two compounds isolated from peony, demonstrated anti-inflammatory properties in animal studies. The researchers found that paeonin inhibited experimentally-induced arthritis in rats. Additionally, paeoniflorin was shown to inhibit contact hypersensitivity in mice and anaphylactic skin reactions in guinea pigs (allergic inflammatory responses). (1) A second animal study confirmed that peony exhibited anti-inflammatory properties, and found that peony showed additive anti-inflammatory effects when combined with the herb cnidium (Ligusticum Wallichi root). (2)
Peony has also demonstrated immune system effects that support its anti-inflammatory effects. Researchers isolated two polysaccharides, Peona SA and Peonan SB, that were shown to stimulate the reticulo-endothelial system (RES), the network of tissue macrophages that act as scavengers to rid the body of toxic or noxious agents. (3) These polysaccharides were also found to inhibit “complement,” a group of proteins that play a role in the inflammatory response. (4)
In 1990 Chinese researchers conducted preliminary pharmacological studies on a Chinese herbal combination that included Peony. While the entire combination exhibited anti-inflammatory effects, Peony in particular was found to have an analgesic effect. (5) This effect was further demonstrated recently when Chinese scientists discovered that paeoniflorin, one of the active principles of peony root, exerted an analgesic effect in mice via opioid receptors. (6)
Chinese researchers chemically induced arthritis in rats and then treated them with TGP. Three groups of animals were given either 10, 50 or 100 mg/kg of TGP per day. A fourth control group was treated with Lobenzarit disodium, an approved RA treatment, at 50 mg/kg. Evaluation at 1 to 7 days found that primary inflammation was reduced in animals receiving TGP. In addition to its anti-inflammatory effects, TGP was observed to support immune response by normalizing T-helper/T-suppressor cells. TGP also reduced secretion of Interleukin-1 (IL-1) and tumor necrosis factor (TNF) two pivotal cytokines involved in the pathogenesis of RA. Additionally, TGP reduced synovial levels of PGE, an arachidonic acid metabolite responsible for much of the pain and swelling associated with RA. These findings suggest that TGP may be of therapeutic benefit in other autoimmune conditions such as rheumatoid arthritis.
Human Clinical Trials
Human clinical trials in China support the therapeutic benefits of peony extract for rheumatoid arthritis. In one study, 120 patients with rheumatoid arthritis received either a standardized peony extract (TGP) or methotrexate (MTX) an established treatment for RA that was used as a control. After four weeks of treatment, both TGP and MTX were found to be equally effective. (7)
These results were replicated in a second study involving 263 Rheumatoid arthritis patients given doses of standardized TGP peony extract. In this study 142 patients again were given MTX to serve as controls. After four weeks, 77 percent of patients receiving the peony extract reported improvement of symptoms – a figure again matched by the methotrexate control group. One difference that did surface in this second study was that peony extract had a normalizing effect on immune function. Additionally, patients receiving the peony extract reported noticeable improvements in both the quality of life and increases in physical strength. These improvements were not reported by patients receiving MTX.
Stage III Clinical Study
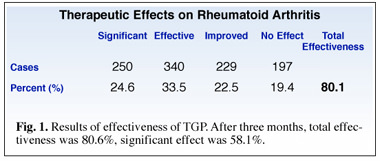
In a multi-center open trial, conducted in China from February 1997 to November 1997, 1,016 subjects were given 600 mg/day of TGP for six months. At three months 80.6% of the subjects reported improvements that included lessened morning stiffness, reduced joint swelling and tenderness, enhanced gripping power, and decreased Rheumatoid factor. 126 subjects continued to take TGP for six months, at which time the percentage of positive respondents had increased to 86.5%, suggesting that TPG’s therapeutic effects on rheumatoid arthritis are cumulative, and increase in effectiveness with extended treatment. (Fig. 1)
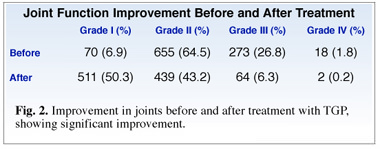
Of special note is that following TGP treatment, 40% of the patients reported significantly improved joint function in addition to relief of pain (Fig. 2).
Safety of TGP
Safety and tolerance studies have found TGP to be safe and free of serious side effects. Medical safety and tolerance tests involving 450 patients found that tolerance of TGP was superior to MTX. Incidence of adverse reactions, defined as gastrointestinal symptoms, poor appetite, nausea, constipation, diarrhea and stomatitis (mouth ulcers), were only 13.73% with TGP, compared to 56.67% for MTX. Total side effects in the Stage III clinical study were very low (13.4%) and most occurred in early treatment stage (within one month).
The most common symptom reported was loose stool, which was self-correcting and required no special attention. Adverse reactions for those treated for six months were similar to those for three months. There were no indications of liver or kidney dysfunction.
References
1. Yamahara, J. et al. Biologically active principles of crude drugs. II Anti-allergic principles in “Shoseiryu-to” anti-inflammatory properties of paeoniflorin and its derivatives. J. Pharm Dyn 1982;5:921-29.
2. Kojima, S. et al. Inhibitory effects of traditional Chinese medicine shimotsu-to and its included fractions on adjuvant-induced chronic inflammation of mice. Biol Pharm Bull 1996;19(1):47-52.
3. Dean, W. English, J. ƒlie Metchnikoff, in Breakthrough Probiotic Clinically Proven To Support Gastrointestinal Health. Vitamin Research News, 1998, 12:4, 1-12.
4. Tomoda, M. et al. Characterization of a neutral and an acidic polysaccharide having immunological activities from the root of Paeonia lactiflora. Biol Pharm. Bull. 1993;16(12):1207-1210.
5. Huang L, et al. A preliminary study on the pharmacology of the compound prescription huangqin tang and its component drugs. Zhongguo Zhong Yao Za Zhi. 1990 Feb;15(2):115-7, 128. [in Chinese]
6. Tsai, H-Y., et al. Effects of paeoniflorin on the formalin-induced nociceptive behavior in mice. Journal of Ethnopharmacology 2001;75:267-71.
7. Unpublished data from Clinical and Safety trials conducted in 1997 by Hui Medical University, Clinical Pharmacology Research Institute. Clinical Trial Centers participating in trials included: Shanhai Tongren Hospital; Guang Hua Hospital; Nanjing Guolou Hospital; Shandong Medical University Hospital; An Hui State Hospital.












[…] that help balance an out-of-control immune system. Numerous studies have shown remarkable positive results when patients are treated with paeoniflorin. Paeoniflorin also has anti-inflammatory and analgesic […]