Researchers from the University of Southern California and the Oak Crest Institute of Science have discovered the link between antibiotics and bacterial biofilm formation leading to chronic lung, sinus and ear infections. The study results, published in the current issue of PLOS ONE, illustrate how bacterial biofilms can actually thrive, rather than decrease, when given low doses of antibiotics.
“This research addresses the long standing issues surrounding chronic ear infections and why some children experience repeated ear infections even after antibiotic treatment,” said Paul Webster, PhD, lead author, senior staff scientist at USC and senior faculty at the Oak Crest Institute of Science. “Once the biofilm forms, it becomes stronger with each treatment of antibiotics.”
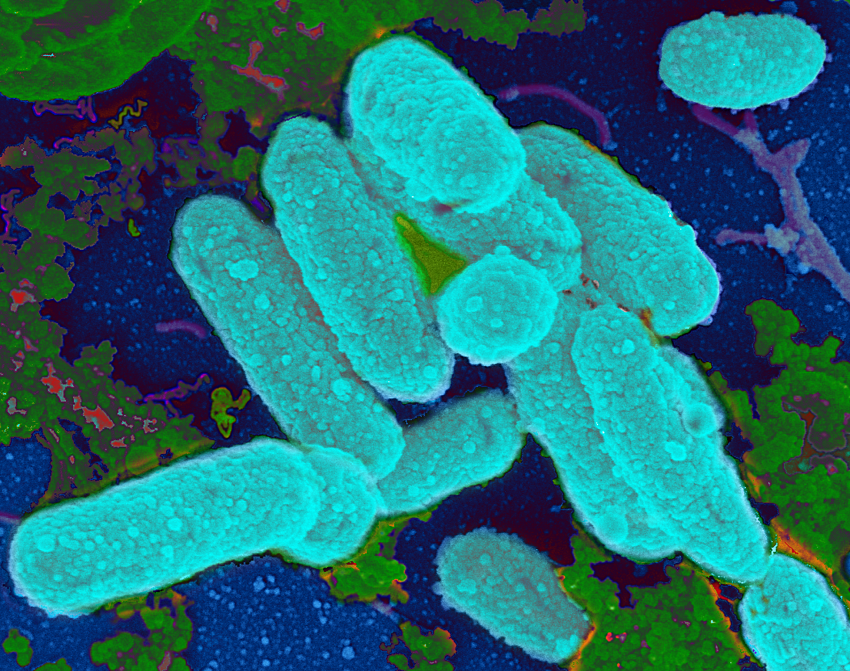
During the study, non-typeable Haemophilus influenzae (NTHi) bacteria (see image above) a common pathogen of humans was exposed to non-lethal doses of ampicillin, a class of antibiotics commonly used to treat respiratory, sinus and ear infections, or other beta-lactam antibiotics. The dose of the antibiotic was not enough to kill the bacteria which allowed the bacteria to react to the antibiotic by producing glycogen, a complex sugar often used by bacteria as a food source, to produce stronger biofilms when grown in the laboratory.
Biofilms are highly structured communities of microorganisms that attach to one another and to surfaces. The microorganisms group together and form a slimy, polysaccharide cover. This layer is highly protective for the organisms within it, and when new bacteria are produced they stay within the slimy layer. With the introduction of antibiotic-produced glycogen, the biofilms have an almost endless food source that can be used once antibiotic exposure has ended.
There are currently no approved treatments for biofilm-related infections. Therefore, bacteria forced into forming stronger biofilms will become more difficult to treat and will cause more severe chronic infections.
Adults will suffer protracted lung infections as the bacteria hunker down into their protective slime, and children will have repeated ear infections. What may appear to be antibiotic resistance when an infection does not clear up may actually be biofilms at work.
Webster believes modern medicine needs to find ways of detecting and treating biofilm infections before the bacteria are able to form these protective structures. The difficulties of treating biofilm infections, which can be up to 1,000 times more resistant to antibiotics, have prompted some physicians to propose a gradual move away from traditional antibiotic treatments and toward non-antibiotic therapies.
“If antibiotics are to continue to be relevant for treating bacterial infections it is important that their effects on biofilms be explored,” says Dr. Webster.
“One step in this direction would be to develop routine screening methods to test the effects of antibiotics on in vitro formed biofilms.”
Source: Siva Wu, Xiaojin Li, Manjula Gunawardana, Kathleen Maguire, Debbie Guerrero-Given, Christoph Schaudinn, Charles Wang, Marc M. Baum, Paul Webster. Beta- Lactam Antibiotics Stimulate Biofilm Formation in Non-Typeable Haemophilus influenzae by Up-Regulating Carbohydrate Metabolism. PLoS ONE, 2014; 9 (7): e99204 DOI: 10.1371/journal.pone.0099204