 A team of researchers from The University of Manchester report that mefenamic acid, an anti-inflammatory drug commonly used to treat menstrual pain, has successfully reversed symptoms of Alzheimer’s disease in mice. The researchers, led by Dr David Brough, discovered that the anti-inflammatory drug was able to completely reverse memory loss and brain inflammation, both hallmark changes of Alzheimer’s disease, in mice specially bred to develop Alzheimer’s.
A team of researchers from The University of Manchester report that mefenamic acid, an anti-inflammatory drug commonly used to treat menstrual pain, has successfully reversed symptoms of Alzheimer’s disease in mice. The researchers, led by Dr David Brough, discovered that the anti-inflammatory drug was able to completely reverse memory loss and brain inflammation, both hallmark changes of Alzheimer’s disease, in mice specially bred to develop Alzheimer’s.
Mefenamic acid was shown to work by targeting an inflammatory pathway known as NLRP3 inflammasome, which is known to damage brain cells. The results suggest that inflammation causes the neurological disease to get worse, and treatment of the inflammation may reduce its effects.
Though this is the first time a drug has been shown to target this inflammatory pathway Dr Brough cautions that more research is needed to identify its impact on humans, and the long-term implications of its use.
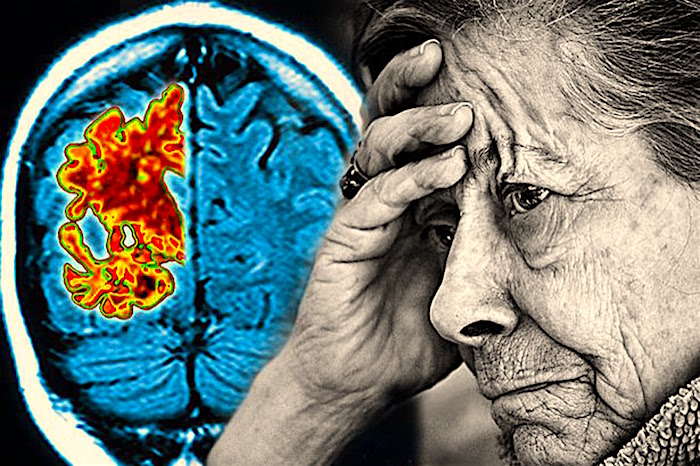
An estimated 5.4 million Americans have Alzheimer’s disease, which gets worse over time, affecting many aspects of their lives, including the ability to remember, think and make decisions.
In the study transgenic mice that develop symptoms of Alzheimer’s disease were used. One group of 10 mice was treated with mefenamic acid, and 10 mice were treated in the same way with a placebo.
The mice were treated at a time when they had developed memory problems and the drug was given to them by a mini-pump implanted under the skin for one month.
Memory loss was completely reversed back to the levels seen in mice without the disease.
Dr Brough said: “There is experimental evidence now to strongly suggest that inflammation in the brain makes Alzheimer’s disease worse.
“Our research shows for the first time that mefenamic acid, a simple Non-Steroidal Anti Inflammatory Drug can target an important inflammatory pathway called the NLRP3 inflammasome, which damages brain cells.”
He added: “Until now, no drug has been available to target this pathway, so we are very excited by this result.
“However, much more work needs to be done until we can say with certainty that it will tackle the disease in humans as mouse models don’t always faithfully replicate the human disease.
“Because this drug is already available and the toxicity and pharmacokinetics of the drug is known, the time for it to reach patients should, in theory, be shorter than if we were developing completely new drugs.
“We are now preparing applications to perform early phase II trials to determine a proof-of-concept that the molecules have an effect on neuroinflammation in humans.”
Dr Doug Brown, Director of Research and Development at Alzheimer’s Society, said: “Testing drugs already in use for other conditions is a priority for Alzheimer’s Society – it could allow us to shortcut the fifteen years or so needed to develop a new dementia drug from scratch.
“These promising lab results identify a class of existing drugs that have potential to treat Alzheimer’s disease by blocking a particular part of the immune response. However, these drugs are not without side effects and should not be taken for Alzheimer’s disease at this stage – studies in people are needed first.”
Source: Fenamate NSAIDs inhibit the NLRP3 inflammasome and 2 protect against Alzheimer’s disease in rodent models, Nature Communications. DOI: 10.1038/NCOMMS12504