Scientists have long studied sleep, a phenomenon that appears to be fundamental for life, yet one that in many ways remains mysterious. Almost every known animal sleeps or exhibits some form of sleeplike behavior. Without enough of it, serious consequences ensue. In humans, chronic insufficient sleep is associated with heart disease, type 2 diabetes, cancer, obesity, depression and many other conditions.
One in three American adults gets less than the recommended seven hours of sleep per night, according to the U.S. Centers for Disease Control and Prevention, and insufficient sleep is a normal part of life for many around the world.
Previous research has shown that prolonged, total sleep restriction can lead to premature death in animal models. While it has been reported in humans only anecdotally, a widely cited study in rats conducted by Chicago-based researchers in 1983 showed that a total lack of sleep inevitably leads to death. Yet, despite decades of study, a central question has remained unsolved: why do animals die when they don’t sleep?
In attempts to answer how sleep deprivation culminates in death, most research efforts have focused on the brain, where sleep originates, but none have yielded conclusive results.
Gut Damage Linked to Premature Death From Sleep-Loss
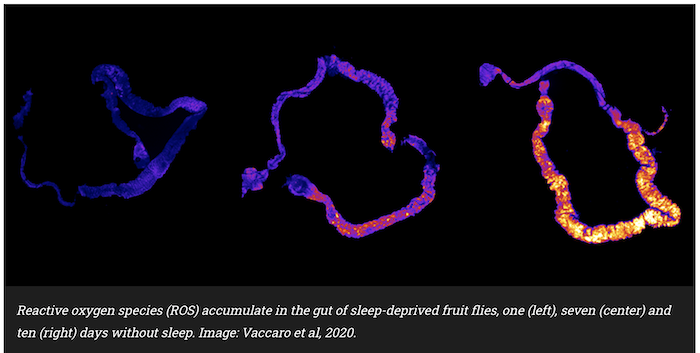
Now, Harvard Medical School neuroscientists have identified an unexpected link between sleep deprivation and premature death. In a study of sleep-deprived fruit flies, researchers discovered that death from sleep deprivation is always preceded by the accumulation of molecules known as reactive oxidative species (ROS) in the gut.
When fruit flies were given antioxidant compounds that neutralize and clear ROS from the gut, sleep-deprived flies remained active and had normal lifespans. Additional experiments in mice confirmed that ROS accumulate in the gut when sleep is insufficient.
The findings, published in the June 4, 2020 issue of Cell, suggest the possibility that animals can indeed survive without sleep under certain circumstances. The results open new avenues of study to understand the full consequences of insufficient sleep and may someday help to counteract the harmful effects of sleep loss in humans, the authors said.
“We took an unbiased approach and searched throughout the body for indicators of damage from sleep deprivation. We were surprised to find it was the gut that plays a key role in causing death,” said senior study author Dragana Rogulja, assistant professor of neurobiology in the Blavatnik Institute at HMS.
“Even more surprising, we found that premature death could be prevented. Each morning, we would all gather around to look at the flies, with disbelief to be honest. What we saw is that every time we could neutralize ROS in the gut, we could rescue the flies,” Rogulja said.
Gut Accumulation of Reactive Oxidative Species
Spearheaded by study co-first authors Alexandra Vaccaro and Yosef Kaplan Dor, both research fellows in neurobiology at HMS, the team carried out a series of experiments in fruit flies, which share many sleep-regulating genes with humans, to search for signs of damage caused by sleep deprivation throughout the body.
To monitor sleep, the researchers used infrared beams to constantly track the movement of flies housed in individual tubes. They found that flies can sleep through physical shaking, so the team turned to more sophisticated methods. They genetically manipulated fruit flies to express a heat-sensitive protein in specific neurons, the activity of which are known to suppress sleep. When flies were housed at 29 degrees C (84 degrees F), the protein induced neurons to remain constantly active, thus preventing the flies from sleeping.
Death rates in sleep-deprived fruit flies spiked after 10 days, and all were dead by around day 20. Control flies with normal sleep lived up to approximately 40 days in the same environmental conditions.
Because mortality increased around day 10, the researchers looked for markers of cell damage on that and preceding days. Most tissues, including in the brain, were indistinguishable between sleep-deprived and non-deprived flies, with one notable exception.
The guts of sleep-deprived flies had a dramatic buildup of ROS – highly reactive, oxygen-containing molecules that in large amounts can damage DNA and other components within cells, leading to cell death. The accumulation of ROS peaked around day 10 of sleep deprivation, and when deprivation was stopped, ROS levels decreased.
Additional experiments confirmed that ROS builds up in the gut of only those animals that experienced sustained sleep loss, and that the gut is indeed the main source of this apparently lethal ROS.
“We found that sleep-deprived flies were dying at the same pace, every time, and when we looked at markers of cell damage and death, the one tissue that really stood out was the gut,” Vaccaro said. “I remember when we did the first experiment, you could immediately tell under the microscope that there was a striking difference. That almost never happens in lab research.”
The team also examined whether ROS accumulation occurs in other species by using gentle, continuous mechanical stimulation to keep mice awake for up to five days. Compared to control animals, sleep-deprived mice had elevated ROS levels in the small and large intestines but not in other organs, a finding consistent with the observations in flies.
Common Supplements Prevent Sleep-Loss Induced Death
To find out if ROS in the gut play a causal role in sleep deprivation-induced death, the researchers looked at whether preventing ROS accumulation could prolong survival.
They tested dozens of compounds with antioxidant properties known to neutralize ROS and identified 11 that, when given as a food supplement, allowed sleep-deprived flies to have a normal or near-normal lifespan. These compounds, such as melatonin, lipoic acid and NAD, were particularly effective at clearing ROS from the gut. Notably, supplementation did not extend the lifespan of non-deprived flies.

The role of ROS removal in preventing death was further confirmed by experiments in which flies were genetically manipulated to overproduce antioxidant enzymes in their guts. These flies had normal to near-normal lifespans when sleep-deprived, which was not the case for control flies that overproduced antioxidant enzymes in the nervous system.
The results demonstrate that ROS buildup in the gut plays a central role in causing premature death from sleep deprivation, the researchers said, but cautioned that many questions remain unanswered.
“We still don’t know why sleep loss causes ROS accumulation in the gut, and why this is lethal,” said Kaplan Dor. “Sleep deprivation could directly affect the gut, but the trigger may also originate in the brain. Similarly, death could be due to damage in the gut or because high levels of ROS have systemic effects, or some combination of these.”
Insufficient sleep is known to interfere with the body’s hunger signaling pathways, so the team also measured fruit fly food intake to analyze whether there were potential associations between feeding and death. They found that some sleep-deprived flies ate more throughout the day compared with non-deprived controls. However, restricting access to food had no effect on survival, suggesting that factors beyond food intake are involved.
The researchers are now working to identify the biological pathways that lead to ROS accumulation in the gut and subsequent physiological disruptions.
“So many of us are chronically sleep deprived. Even if we know staying up late every night is bad, we still do it,” Rogulja said. “We believe we’ve identified a central issue that, when eliminated, allows for survival without sleep, at least in fruit flies.”
Source: Alexandra Vaccaro, Yosef Kaplan Dor, Keishi Nambara, Elizabeth A. Pollina, Cindy Lin, Michael E. Greenberg, Dragana Rogulja. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell, 2020; DOI: 10.1016/j.cell.2020.04.049