Researchers have developed tiny motorized molecules that can be activated by light to target and drill through highly antibiotic resistant bacteria, killing them within minutes. Molecular drills have the ability to target and destroy deadly bacteria that have become resistance to nearly all antibiotics. In some cases, the drills make the antibiotics effective once again.
Researchers at Rice University, Texas A&M University, Biola University and Durham (U.K.) University showed that motorized molecules developed in the Rice lab of chemist James Tour are effective at killing antibiotic-resistant microbes within minutes.
“These superbugs could kill 10 million people a year by 2050, way overtaking cancer,” Tour said. “These are nightmare bacteria; they don’t respond to anything.”
The motors target the bacteria and, once activated with light, burrow through their exteriors.
While bacteria can evolve to resist antibiotics by locking the antibiotics out, they have no defense against molecular drills. Antibiotics able to get through openings made by the drills are once again lethal to the bacteria.
Tour and Robert Pal, a Royal Society University Research Fellow at Durham and co-author of the new paper, introduced the molecular drills for boring through cells in 2017.
The drills are paddlelike molecules that can be prompted to spin at 3 million rotations per second when activated with light.
Tests by the Texas A&M lab of lead scientist Jeffrey Cirillo and former Rice researcher Richard Gunasekera, now at Biola, effectively killed Klebsiella pneumoniae within minutes. Microscopic images of targeted bacteria showed where motors had drilled through cell walls.
The transmission electron microscope image below shows the damage caused to a Klebsiella pneumoniae bacteria exposed to the motorized molecular nanomachines.
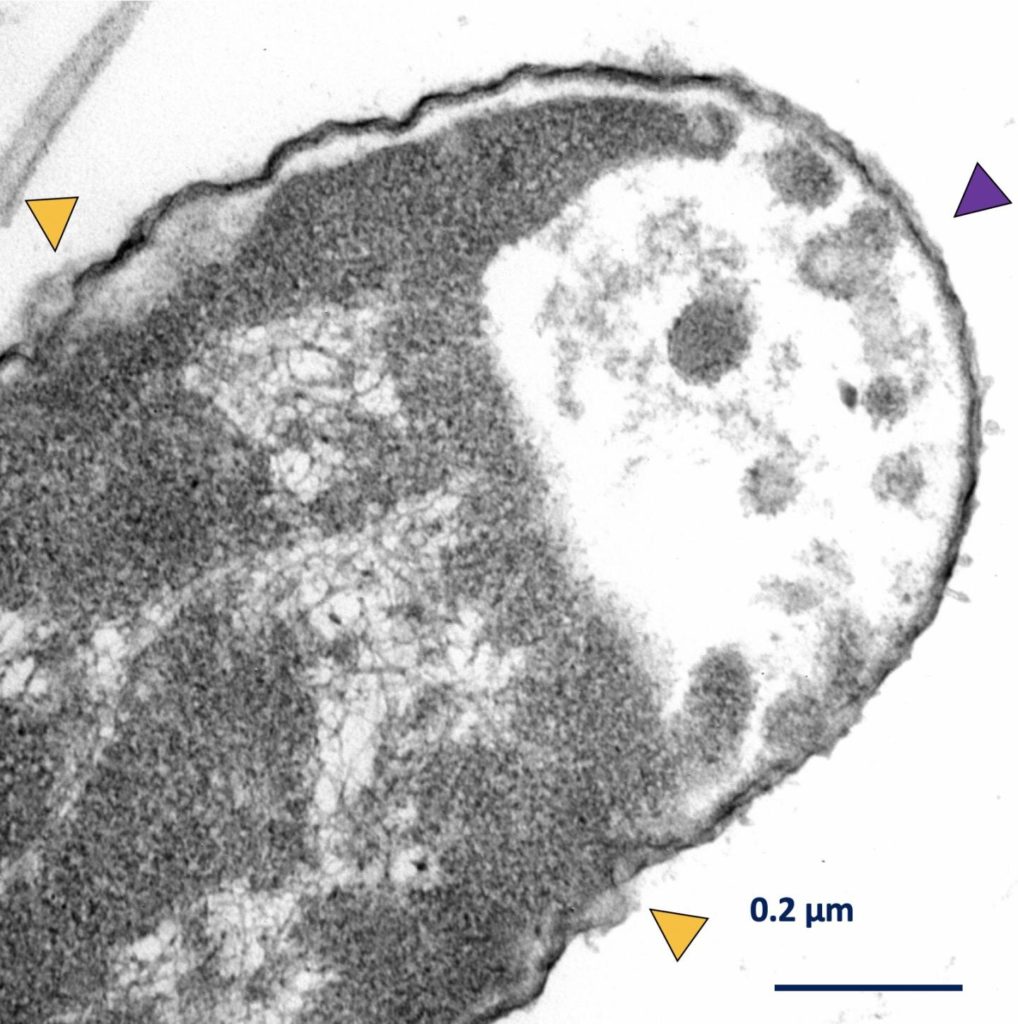 The yellow arrows show areas of cell wall disruptions, while the purple arrow shows where cytoplasm has escaped from the cell. Credit: Don Thushara Galbadage/Texas A&M
The yellow arrows show areas of cell wall disruptions, while the purple arrow shows where cytoplasm has escaped from the cell. Credit: Don Thushara Galbadage/Texas A&M
“Bacteria don’t just have a lipid bilayer,” Tour said. “They have two bilayers and proteins with sugars that interlink them, so things don’t normally get through these very robust cell walls.”
“That’s why these bacteria are so hard to kill. But they have no way to defend against a machine like these molecular drills, since this is a mechanical action and not a chemical effect.”
The motors also increased the susceptibility of K. pneumonia to meropenem, an antibacterial drug to which the bacteria had developed resistance. “Sometimes, when the bacteria figures out a drug, it doesn’t let it in,” Tour said. “Other times, bacteria defeat the drug by letting it in and deactivating it.”
He said meropenem is an example of the former. “Now we can get it through the cell wall,” Tour said. “This can breathe new life into ineffective antibiotics by using them in combination with the molecular drills.”
Gunasekera said bacterial colonies targeted with a small concentration of nanomachines alone killed up to 17% of cells, but that increased to 65% with the addition of meropenem. After further balancing motors and the antibiotic, the researchers were able to kill 94% of the pneumonia-causing pathogen.
Tour said the nanomachines may see their most immediate impact in treating skin, wound, catheter or implant infections caused by bacteria—like staphylococcus aureus MRSA, klebsiella or pseudomonas—and intestinal infections.
“On the skin, in the lungs or in the GI tract, wherever we can introduce a light source, we can attack these bacteria,” he said. “Or one could have the blood flow through a light-containing external box and then back into the body to kill blood-borne bacteria.”
“We are very much interested in treating wound and implant infections initially,” Cirillo said. “But we have ways to deliver these wavelengths of light to lung infections that cause numerous mortalities from pneumonia, cystic fibrosis and tuberculosis, so we will also be developing respiratory infection treatments.”
Gunasekera noted bladder-borne bacteria that cause urinary tract infections may also be targeted.
The paper is one of two published by the Tour lab this week that advance the ability of microscopic nanomachines to treat disease. In the other, which appears in ACS Applied Materials Interfaces, researchers at Rice and the University of Texas MD Anderson Cancer Center targeted and attacked lab samples of pancreatic cancer cells with machines that respond to visible rather than the previously used ultraviolet light. “This is another big advance, since visible light will not cause as much damage to the surrounding cells,” Tour said.
Source: Thushara Galbadage, Dongdong Liu, Lawrence B. Alemany, Robert Pal, James M. Tour, Richard S. Gunasekera, Jeffrey D. Cirillo. Molecular Nanomachines Disrupt Bacterial Cell Wall, Increasing Sensitivity of Extensively Drug-Resistant Klebsiella pneumoniae to Meropenem. ACS Nano, 2019; DOI: 10.1021/acsnano.9b07836